The 2023 Nobel Prize in Physiology or Medicine was awarded jointly to Katalin Karikó and Drew Weissman for their groundbreaking research on nucleoside base modifications. These modifications paved the way for the development of highly effective mRNA vaccines against COVID-19. Their discoveries played a crucial role in the rapid creation of vaccines during the pandemic, revolutionizing our comprehension of mRNA’s interaction with the immune system and significantly impacting global health during this unprecedented crisis.
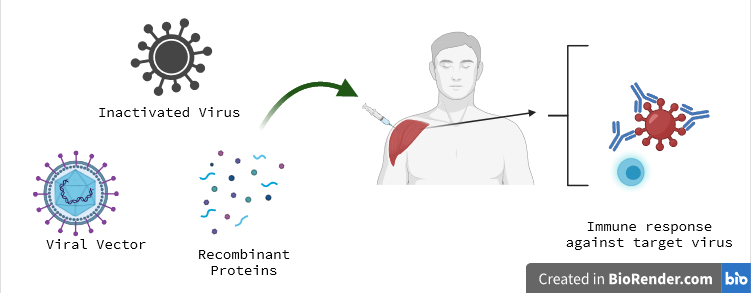
Conventional vaccines before pandemic.
Vaccination initiates the body’s immune response against specific pathogens, providing an advantage in combating diseases upon future exposure. Traditional vaccines, such as those for polio, measles, and yellow fever, have relied on weakened or killed viruses. Max Theiler was honored with the Nobel Prize in Physiology or Medicine in 1951 for his work on the yellow fever vaccine.
Advancements in molecular biology have led to the development of vaccines based on individual viral components rather than entire viruses. These vaccines utilize segments of viral genetic material, typically encoding surface proteins, to trigger the production of antibodies that block the virus. Examples include vaccines against hepatitis B and human papillomavirus. Alternatively, viral genetic material can be inserted into a harmless carrier virus, known as a “vector,” as seen in Ebola vaccines. When administered, vector vaccines prompt our cells to produce the targeted viral protein, eliciting an immune response.
The production of whole virus, protein based, and vector based vaccines typically relies on large-scale cell cultures. However, this resource-intensive process poses limitations on rapid vaccine development during outbreaks and pandemics. Consequently, researchers have endeavored to develop cell culture independent vaccine technologies, though this has presented significant challenges.
mRNA Vaccines: Pioneering Potential
In our cells, DNA carries genetic information that is translated into messenger RNA (mRNA), serving as a blueprint for protein synthesis. The 1980s saw the introduction of efficient techniques for mRNA production outside of cell culture, known as in vitro transcription, marking a significant milestone in molecular biology advancement. This breakthrough spurred the exploration of mRNA technologies for various applications, including vaccines and therapeutics, although hurdles needed to be overcome. Challenges arose as in vitro-transcribed mRNA was deemed unstable and difficult to deliver, necessitating the development of sophisticated lipid carrier systems for mRNA encapsulation. Additionally, its production triggered inflammatory reactions, dampening initial enthusiasm for its clinical use.
Despite these challenges, Hungarian biochemist Katalin Karikó remained undeterred in her pursuit of harnessing mRNA for therapy. During the early 1990s, while serving as an assistant professor at the University of Pennsylvania, Karikó persisted in her vision despite facing skepticism from research funders. At the same institution, immunologist Drew Weissman, intrigued by dendritic cells’ role in immune surveillance and vaccine induced immune responses, became Karikó’s new collaborator. Their partnership, fueled by innovative ideas, delved into understanding how different RNA types interacted with the immune system, laying the groundwork for the future of mRNA-based therapeutics and vaccines.
Experimental Results: Modified mRNA Variants
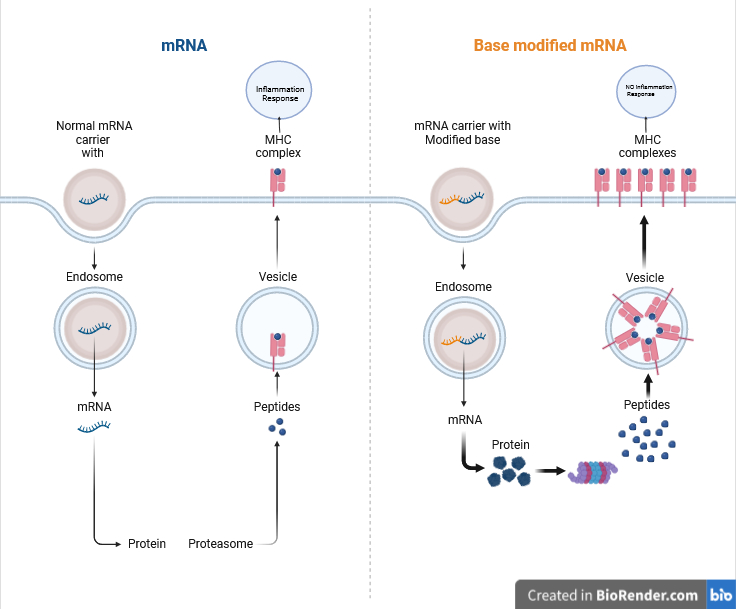
Karikó and Weissman observed a distinctive reaction of dendritic cells to in vitro transcribed mRNA, prompting their activation and the release of inflammatory signals. This reaction raised a fundamental question: why did mRNA from mammalian cells not elicit the same response? The duo speculated that specific characteristics must differentiate between the two mRNA types.
RNA, composed of four bases A, U, G, and C corresponds to the genetic code’s DNA letters: A, T, G, and C. Karikó and Weissman recognized that RNA from mammalian cells often undergoes chemical modifications absent in in vitro transcribed mRNA. They hypothesized that these modifications, or lack thereof, might account for the differing immune responses. To test their theory, they engineered various mRNA variants, each with distinct base modifications, and introduced them to dendritic cells. The outcome was profound: the inflammatory reaction significantly diminished when mRNA contained base alterations. This revelation revolutionized our comprehension of cellular recognition and response to diverse mRNA forms.
Realizing the far reaching implications of their findings for mRNA based therapy, Karikó and Weissman swiftly grasped the significance of their discovery. Their groundbreaking results, unveiled in 2005, a decade and a half before the onset of the COVID-19 pandemic, heralded a paradigm shift in the therapeutic potential of mRNA.
In subsequent research articles released in 2008 and 2010, Karikó and Weissman demonstrated that incorporating base modifications into mRNA significantly enhanced protein synthesis compared to unmodified mRNA delivery. This improvement stemmed from the decreased activation of an enzyme responsible for regulating protein production. By showcasing that base modifications not only minimized inflammatory reactions but also boosted protein output, Karikó and Weissman successfully overcame major hurdles obstructing the clinical utilization of mRNA
Beyond COVID-19: The Versatility of mRNA Vaccines
In 2010, the spotlight turned to mRNA technology as several companies delved into its development. Their focus? Crafting vaccines against the Zika virus and MERS-CoV, a close relative of SARS-CoV-2. Then, with the onset of the COVID-19 pandemic, the race was on. Two groundbreaking mRNA vaccines, engineered with base modifications and encoding the SARS-CoV-2 surface protein, emerged at an unprecedented pace. By December 2020, both were approved, boasting remarkable efficacy rates of approximately 95%.
The remarkable agility and rapidity exhibited by mRNA vaccines in their development herald a new era in vaccine creation, extending far beyond COVID-19. This innovative platform holds promise for combating a spectrum of infectious diseases in the future. Moreover, the horizon gleams with potential for mRNA technology to revolutionize therapeutic protein delivery, offering potential treatments for various cancer types.
Amidst the COVID-19 vaccine landscape, a multitude of other vaccines, employing diverse methodologies, swiftly entered the fray. Together, these efforts culminated in over 13 billion doses administered globally. The impact? Millions of lives saved, and countless others shielded from severe illness, affording societies the chance to resume normalcy.
In this land mark achievement, 2023 Nobel laureates stand as pivotal figures. Through their seminal discoveries elucidating the crucial role of base modifications in mRNA, they have left an indelible mark on history, steering humanity through one of its most daunting health crises.